This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Condensate – its causes, formation and polymerization
The electronics market is dynamic and very agile. Hardly any other industry records such rapid growth rates. The constantly growing demand already ensures a stable workload in the electronics industry for decades. At the same time, this is precisely what leads to more residues from the production process being produced and deposited in the soldering equipment. The condensate residues affect the soldering process. They isolate the areas in the soldering system that are to be heated or cooled and thus influence the heat transfer. They fill exhaust lines and thus affect the amount of heat required for an optimum soldering process. They are corrosive and may damage machine components in the long term. Condensate residues also affect the quality of the finished circuit boards. Depending on what was used in which combination, specific residues remain on the PCB after cleaning and can have negative long-term effects. Cleaning the machines to free them from condensate takes time. This time is no longer available for the production process. If many condensate residues form, the cleaning intervals must be shortened. As a waste product of industry, however, they harm the environment. Contact with condensate residues can affect health and trigger allergic reactions. For this very reason, condensate must be studied in detail and the mechanisms of its formation must be understood.
Hardly anyone is interested in the details of the amber-colored condensate residues in the soldering systems, which become continuously darker with increasing temperature exposure. Needle-shaped crystalline formations also appear in the maintenance process, mostly on the inner walls of the extraction pipes – in the areas with a large temperature gradient. This article focuses on the cause of condensate formation and mechanisms responsible for it. In this way, technologies and processes can be developed to minimize condensate formation from the beginning.
The formation of the condensate in the soldering process is a complex process. Outgassing, chemical reactions and condensation of the volatile low-molecular components play a supporting, but not exclusive role. The production environment, temperature windows for condensate formation, presence of suitable reactants are also just some of the many other factors that contribute to condensate formation. Effects such as a consistent disregard of the laws of thermodynamics in the geometry of the exhaust lines in a soldering machine or a use of materials with a catalyst effect also play an important role. The goal of this article is therefore to provide a basic understanding of the formation mechanisms of condensate in the soldering process and thereby to be able to contribute meaningfully to its minimization.
Condensate Residue – Formation
Since even the best quality of individual components can not prevent all components from completely reacting chemically with each other in the manufacturing process, not only the solder paste, but also the PCB substrate, solder resist and component housings begin to outgas in the soldering system. These gaseous molecules can react with each other and form larger molecules. If the influence of temperature is added and suitable conditions are present, polymerization and crosslinking take place.
Polymerization of Condensate Residue
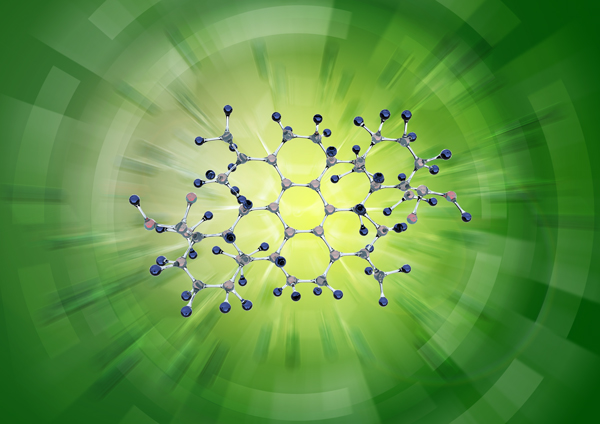
To understand condensate formation, formation mechanisms from polymer chemistry must be used. The polymerization process describes the growth from an organic molecule (monomer) to longer molecular chains (polymers). In this process, different organic molecules can polymerize to form long polymer chains. Polymerization requires reactive bonds, suitable polymerization partners and favorable polymerization conditions (temperature and reaction time). The soldering process provides all this. There, different temperatures exist, a continuous supply of suitable reaction partners is ensured, most of which have the reactive bonds – and if not, thermal degradation makes sure that such bonds can be formed. This is clearly shown by an example of a polymerization reaction of abietic acid from solder paste.
Formation of 5-hydroxy-7-oxodehydroabietic acid polymer by polycondensation of abietic acid (from solder paste).
Crosslinking of Condensate Residue
Crosslinking is the molecular linkage between the single polymer chains formed. The degree of crosslinking significantly determines properties such as strength, dimensional stability, and chemical and thermal resistance. The high temperature in the soldering systems favors the crosslinking reactions, since in addition to the continuous transfer of the reactants, thermal damage to the existing, already crosslinked layer also plays a major role. At high temperatures, the molecular chains of the already crosslinked layer are thermally damaged and new reactive groups are formed, which are available for further crosslinking reactions. Thus, it is not exclusively the amount of reactive low molecular weight compounds produced that is responsible for the degree of crosslinking, but also the duration of the thermal effect on the already polymerized condensate layer. In practice, this continuous post-crosslinking leads to an increased cleaning requirement for the soldering equipment, because this increases both the chemical and the mechanical resistance of the condensate layer.

Condensation Process
The reaction products condense as soon as the ambient temperature has cooled down sufficiently. The larger and bulkier the macromolecules are, the higher the temperature at which they condense. Smaller and lighter macromolecules, on the other hand, condense at a lower temperature. If favorable conditions prevail, such as a suitable temperature window, sufficient time for growth processes and suitable building blocks are available, the formation of crystalline structures takes place. These can grow from the unreacted low-molecular compounds from the PCB substrate or from the components of the solder resist. Also, crystallization processes can start from the reaction products of the flux.
Theoretical analysis of condensate formation is the first step towards a better understanding of the elements and parameters involved and their interaction with each other in the soldering environment. Condensate is more than just a condensed residue from the flux or solder resist on the walls of the soldering machines. When condensate is formed, volatile components of the PCB substrate, soldermask, and solder paste flux must be considered. They can all react with each other in the soldering process to determine condensate growth, condensate quantity and its composition. Understanding about its formation, reaction possibilities, cross-linking and crystallization helps to take appropriate measures. For example, by shifting the temperature outside the process, certain reactions can be stopped or inhibited to slow down the polymerization process. Crystallization processes can be slowed down by selective extraction of the atmosphere at points with preferential outgassing of certain low-molecular compounds, since corresponding reaction partners are only available in a small quantity for a crystallization process.


